Ready to FLIP THE SCRIPT? How to Prescribe IBSRELA

Treatment for IBS-C Is Not One Size Fits All. When Your Patients Aren’t Getting Adequate Relief, Try a Therapy With a Different Mechanism of Action in a Different Class1,2
IBSRELA is dosed as one (1) 50-mg tablet BID
Get Started
Add Transition Pharmacy in Trevose, Pennsylvania as a favorite in your electronic health record system to save time when writing your next prescription.

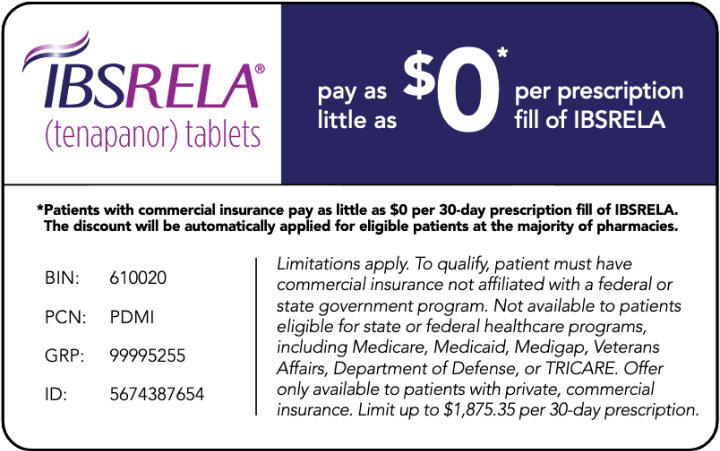
ArdelyxAssistTM Offers Several Programs to Support Patient Affordability
- Commercially insured patients can pay as little as $0† per prescription through the IBSRELA copay program
- ArdelyxAssist offers additional programs for eligible patients who are uninsured or underinsured and are unable to afford IBSRELA
ArdelyxAssist is here to help. Call us at 844-427-7352, option 1, if you have any questions or need support with IBSRELA access or affordability.
To fax a prescription: (877) 765-7664.

Resources for You and Your Patients


References:
1. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(22):6759-6773. 2. IBSRELA [prescribing information]. Waltham, MA: Ardelyx, Inc.; 2022.
INDICATION
IBSRELA (tenapanor) is indicated for the treatment of Irritable Bowel Syndrome with Constipation (IBS-C) in adults.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
IBSRELA is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile rats administration of tenapanor caused deaths presumed to be due to dehydration. Avoid use of IBSRELA in patients 6 years to less than 12 years of age. The safety and effectiveness of IBSRELA have not been established in patients less than 18 years of age.
CONTRAINDICATIONS
- IBSRELA is contraindicated in patients less than 6 years of age due to the risk of serious dehydration.
- IBSRELA is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction.
WARNINGS AND PRECAUTIONS
Risk of Serious Dehydration in Pediatric Patients
- IBSRELA is contraindicated in patients below 6 years of age. The safety and effectiveness of IBSRELA in patients less than 18 years of age have not been established. In young juvenile rats (less than 1 week old; approximate human age equivalent of less than 2 years of age), decreased body weight and deaths occurred, presumed to be due to dehydration, following oral administration of tenapanor. There are no data available in older juvenile rats (human age equivalent 2 years to less than 12 years).
- Avoid the use of IBSRELA in patients 6 years to less than 12 years of age. Although there are no data in older juvenile rats, given the deaths in younger rats and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of IBSRELA in patients 6 years to less than 12 years of age.
Diarrhea
Diarrhea was the most common adverse reaction in two randomized, double-blind, placebo-controlled trials of IBS-C. Severe diarrhea was reported in 2.5% of IBSRELA-treated patients. If severe diarrhea occurs, suspend dosing and rehydrate patient.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions in IBSRELA-treated patients (incidence ≥2% and greater than placebo) were: diarrhea (16% vs 4% placebo), abdominal distension (3% vs <1%), flatulence (3% vs 1%) and dizziness (2% vs <1%).